Structured Illumination Microscopy
Structured illumination microscopy (SIM) is a type of fluorescence microscope that incorporates a structured light generation device, such as a grating, spatial light modulator, or digital micromirror device (DMD), into the illumination path. By altering the spatial structure of the illumination light and using computational reconstruction, SIM overcomes the diffraction limit, achieving a lateral resolution close to or better than 100 nanometers and an axial resolution of about 300 nanometers, which is twice the resolution of traditional microscopes.
Principle:
SIM improves microscope resolution using the Moiré effect, an optical phenomenon where two superimposed patterns with similar spatial frequencies create interference patterns. These interference patterns, called Moiré fringes, have a lower spatial frequency than the original patterns.
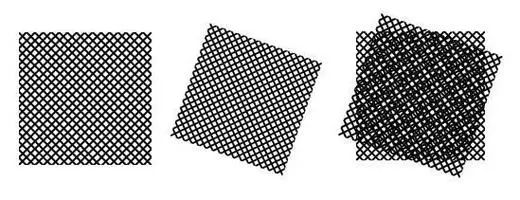
When the human eye cannot distinguish the original grating patterns, it can only see the Moiré fringes. Structured illumination microscopy (SIM) applies this effect to microscopic imaging. By using structured light, such as sinusoidal fringes, to illuminate the sample, it shifts high-frequency information, which is normally inaccessible, to a lower frequency region that the imaging system can capture, thereby enhancing resolution.
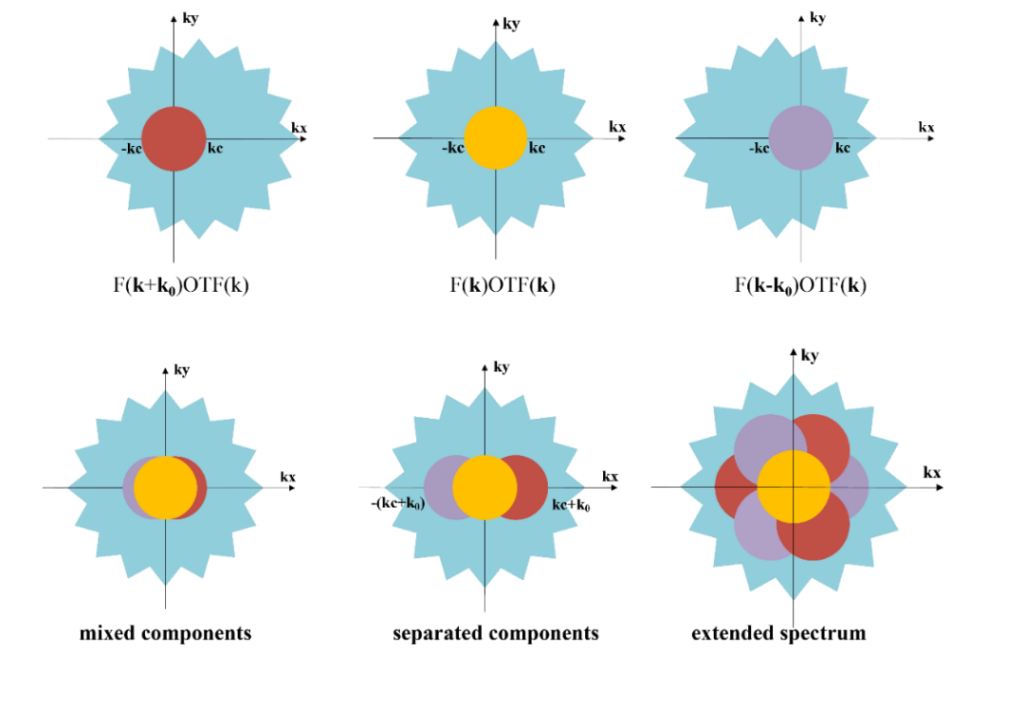
SIM reconstructs the image using algorithms that typically require capturing nine raw images (three different directions, each with three different phases) or fifteen raw images (five different phases for each of the three directions). These images are then processed to separate and optimize the frequency components, improving image quality. The frequency components are moved to their corresponding positions and stitched together to produce a super-resolution image.
Super Resolution Structured Illumination Microscopy
The resolution of traditional microscopes is limited to about 200 nanometers laterally and 500 nanometers axially. This is sufficient for observing overall cell structures and larger organelles but inadequate for resolving fine subcellular structures. Super-resolution microscopy surpasses the resolution limits of optical microscopes, achieving lateral and axial resolutions of tens of nanometers or even smaller, far beyond the diffraction barrier. This enables the observation of more detailed structures. Key techniques include stimulated emission depletion microscopy (STED), photoactivated localization microscopy (PALM), and super-resolution structured illumination microscopy (SR-SIM).
While STED and PALM significantly enhance spatial resolution (achieving resolutions of 20-50 nanometers), they have notable drawbacks: they require intense excitation light, which can cause photobleaching of fluorescent proteins or molecules and generate large amounts of free radicals that damage live cell samples. This makes it difficult to use ordinary fluorescent proteins or molecules for imaging and live cell imaging. In contrast, SR-SIM effectively utilizes photons emitted by fluorescent molecules, significantly reducing the required illumination power. Samples imaged with traditional fluorescence microscopy and confocal microscopy can be used for SIM imaging, making it suitable for long-term imaging and live cell observation.
Optical Sectioning Structured Illumination Microscopy
Optical Sectioning Structured Illumination Microscopy (OS-SIM) is an imaging technique that combines structured illumination microscopy (SIM) technology with optical sectioning capabilities. SIM provides super-resolution imaging by adjusting the illumination angle or focal position to obtain tomographic images of the sample at different depths (Z-axis). The camera captures multiple sets of images with different phases, directions, and depths, with each set representing an optical section of the sample. Using software algorithms, these 2D optical section images are reconstructed into a 3D image, offering a complete, high-resolution view of the internal structure of the sample and constructing a three-dimensional model. This technique is suitable for observing the internal structure of biological samples.
B-SIM298 Structured Illumination Fluorescence Microscope
B-SIM298 is a microscopy system that uses structured illumination to achieve optical sectioning. It features multiple observation and imaging modes, including bright field, fluorescence, phase contrast, and differential interference contrast (DIC) found in wide-field fluorescence microscopes. Additionally, it can eliminate stray light outside the focal plane, enabling three-dimensional reconstruction of cell-level microstructures. The software platform controls the hardware, facilitating image acquisition, processing, and analysis, and can achieve confocal imaging quality.
Features:
1. Motorized research-grade inverted microscope: 12-axis motorization, supports dual-layer independent fluorescence paths, and independent optical imaging and optical control. Supports bright field, phase contrast, differential interference contrast (DIC), relief contrast, polarized light, and fluorescence functions.
2. High numerical aperture, long working distance, apochromatic objective series with air, water, and oil objectives. Various options available to flexibly meet experimental needs.
3. Long-life, high-power independent LED.
4. 1.2-inch, 2048×2048, 4-megapixel back-illuminated USB3.0 sCMOS camera.
5. Independent structured light optical sectioning module: capable of achieving optical resolution of 240nm in the xy direction and 600nm in the z direction.
6. Proprietary software platform: integrates hardware control, image acquisition, image processing, and image analysis functions.
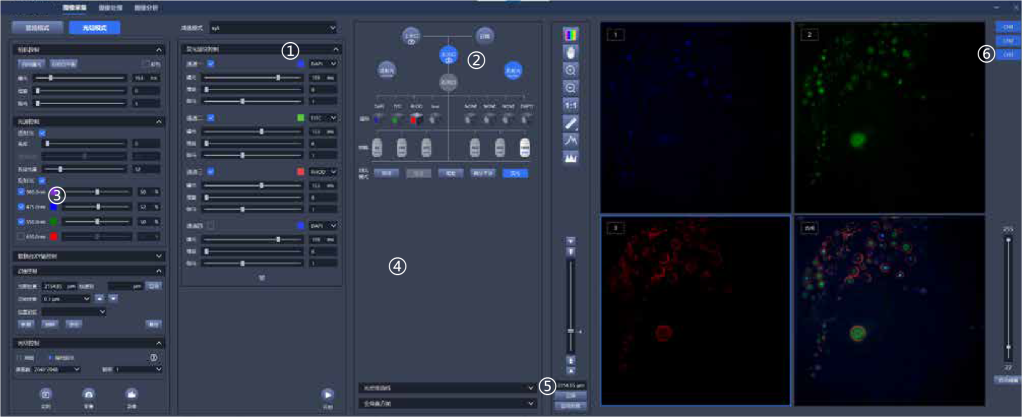
①Five-dimensional sequence acquisition of x, y, z, λ , t, realizes automatic acquisition control.
②Graphical interaction: switch optical port, filter block, objectives with one-click and changing observation mode.
③Electric focusing, electric stage, electric fluorescence filter block, electric condenser, electric light source control, electric light path switching, realizing fully electric 12-axis control.
④Real-time optical density measurement, real-time global histogram display.
⑤Graphical focus control, focus position memory, automatic focus control.
⑥Customize 1-8 channel acquisition, real-time pseudo-color display, arbitrary multi-channel overlay, and real-time display of multi-channel overlay map.
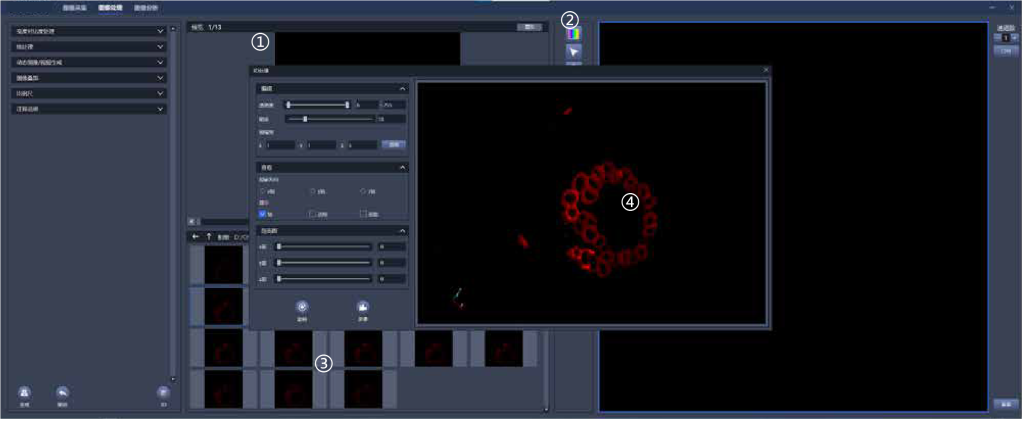
①Real-time display of comparison before and after image processing
②Record image information in detail, including detailed parameters such as acquisition channel, objectives, exposure time, etc.
③Real-time image preview
④3D image reconstruction
Applications:
1. Quantitative, qualitative, localization, and time-lapse analysis
Quantitative: Determine fluorescence intensity by measuring fluorescence brightness.
Qualitative: Identify positive or negative results based on the presence or absence of a fluorescence signal.
Localization: Use multi-channel acquisition and image overlay to determine positional relationships.
Time-lapse: Perform timed acquisition to observe changes in the sample over time.
2. 3D reconstruction
Using thin-layer optical sectioning, true three-dimensional data of the specimen can be obtained. With computer image processing and 3D reconstruction software, this data can be transformed into vivid 3D effects, allowing for flexible and intuitive morphological observation and revealing the spatial relationships of subcellular structures.
3. Cell proliferation and apoptosis
After apoptosis, cell membrane permeability increases, allowing fluorescent dyes to penetrate the cell. By observing at timed intervals and counting cells, one can determine whether the number of cells is increasing or decreasing, thereby assessing the impact on cell proliferation or apoptosis.
4. Intracellular and extracellular communication
FRAP (Fluorescence Recovery After Photobleaching): Used to study the movement and interaction of proteins within the cell by bleaching a specific area and observing the recovery of fluorescence over time.
FLIP (Fluorescence Loss In Photobleaching): Involves continuous bleaching of a specific region and observing the loss of fluorescence in other areas to study the dynamics and connectivity of cellular components.
FRET (Fluorescence Resonance Energy Transfer): A technique to monitor the interaction between two molecules by measuring the energy transfer between two fluorescent proteins.
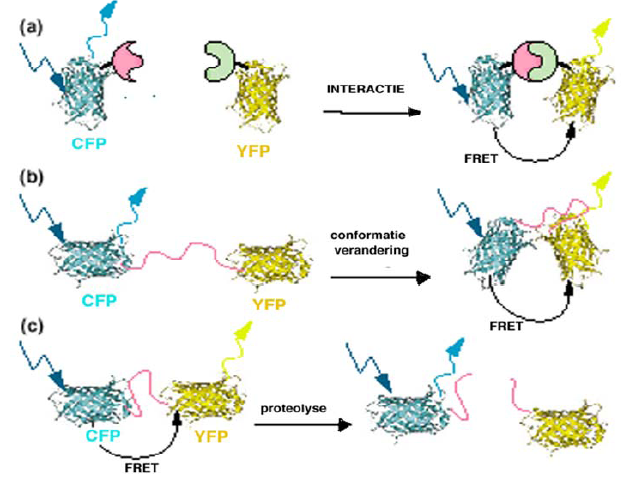
Applications of FRET microscopy
Sample Images

Nucleus – 100X objective
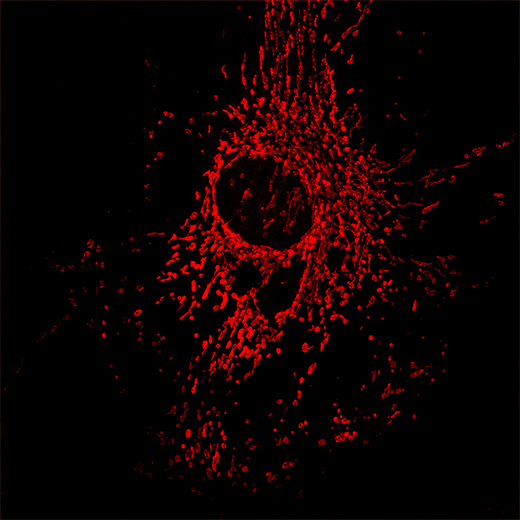
Mitochondria – 100X objective
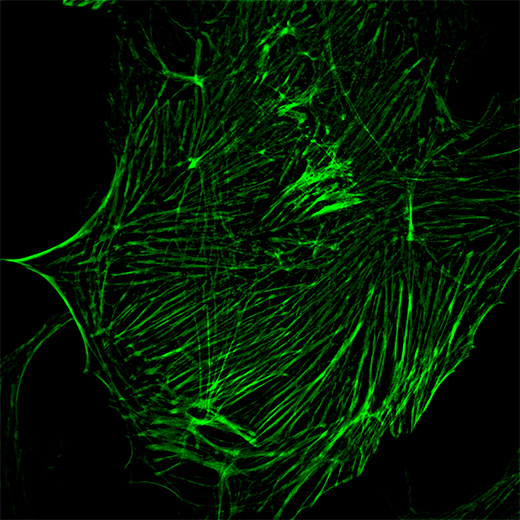
Cytoskeleton – 100X objective
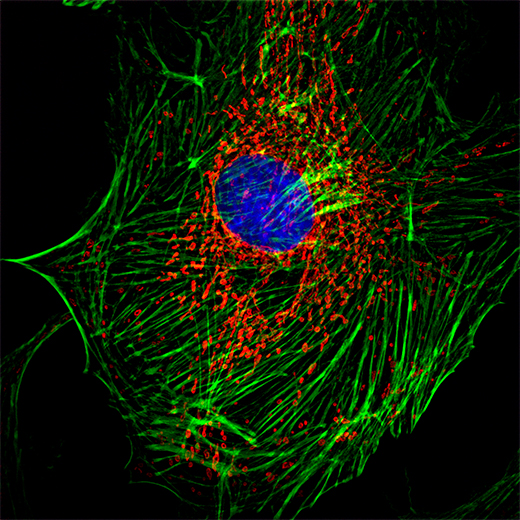
Cell Composite Image – 100X objective
The resources are collected and organized on the Internet, and are only used for learning and communication. If there is any infringement, please contact us to delete.